- [Interview] Cho Byoung-chul, Director of the Lung Cancer Center at Yonsei Cancer Center
- "A historic milestone: Over 1-year OS extension, something I never imagined"
- "OS curve resembles that of ‘immuno바카라 카지노’… Positive drug response expected in many patients"
- "This year’s paper publication boosts chances of NCCN ‘preferred 바카라 카지노’ designation"

[by Ji, Yong Jun] “The combination therapy of ‘lazertinib (marketed in Korea as Leclaza and internationally as Lazcluze) + amivantamab (commercial name Rybrevant) is projected to yield a median overall survival (mOS) of approximately 55 months in patients. Given the current trajectory of clinical outcomes and adoption, this regimen is anticipated to be included as a preferred therapy in the National Comprehensive Cancer Network (NCCN) guidelines within the year.”
Cho Byoung-chul, Director of the Lung Cancer Center at Yonsei Cancer Center and Professor of Oncology at Severance Hospital, a leading figure in the development of the ‘Lazcluze + Rybrevant combination 바카라 카지노,’ shared insights in an interview with <THE BIO on April 1 at the Yonsei Avison Biomedical Research Center in Seodaemun District, Seoul. Professor Cho is the first and corresponding author of the ‘MARIPOSA’ study, a Phase 3 clinical trial evaluating the combination 바카라 카지노. During the interview, Cho discussed the so-called “final piece of the puzzle” that remains for the Lazcluze + Rybrevant combination 바카라 카지노 to complete its clinical validation.
Lazcluze is a third-generation oral (ingestible) epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) developed by the Korean pharmaceutical company Yuhan Corporation. In 2018, Yuhan licensed the technology to Janssen, a subsidiary of Johnson & Johnson (J&J), in a deal valued at USD 1.255 billion (approximately KRW 1.7 trillion). The drug was subsequently approved for use in Korea under the name ‘Leclaza’ and in the United States and Europe under the name ‘Lazcluze.’
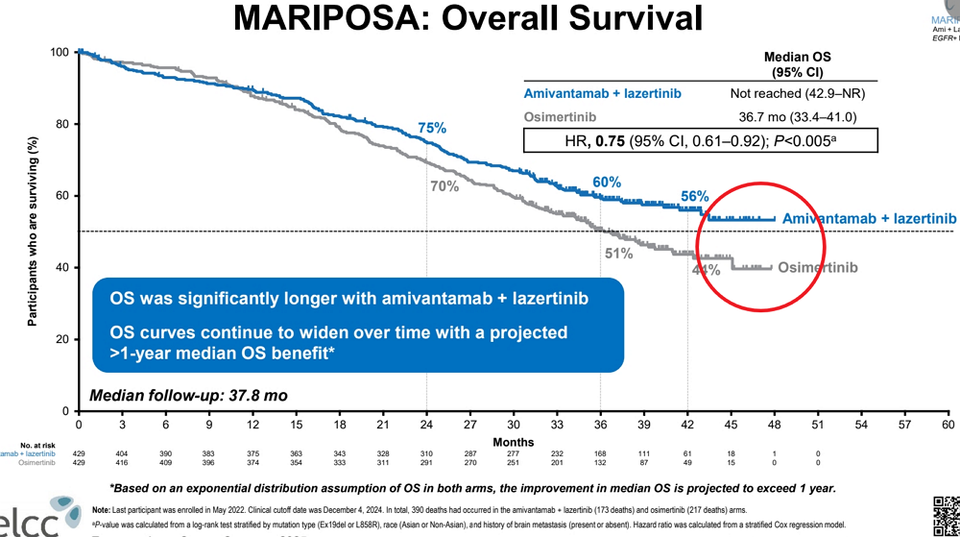
The Lazcluze + Rybrevant combination 바카라 카지노 is garnering significant attention for its potential to make a pivotal historical advancement in the treatment of EGFR-mutated non-small cell lung cancer (NSCLC). On March 26 (local time), findings from the Phase 3 MARIPOSA clinical trial were presented at the European Lung Cancer Congress (ELCC) held in Paris, France. According to the reported data, the Lazcluze + Rybrevant combination 바카라 카지노 demonstrated an mOS of more than 12 months compared to the control group. Specifically, the mOS for the control group, treated with ‘Tagrisso (osimertinib)’, was 36.7 months.
In contrast, the mOS for the Lazcluze + Rybrevant combination 바카라 카지노 has not yet been measured, which is interpreted as indicative of an extended survival duration of at least four years (48 months). Overall survival (OS) period is widely regarded as the most clinically meaningful endpoint for evaluating life expectancy extension in cancer patients. Among the ‘novel lung cancer therapies’ approved to date, only two treatments have demonstrated an OS extension benefit exceeding 12 months in the past century: ‘Keytruda,’ an immuno바카라 카지노 developed by the multinational pharmaceutical company MSD (Merck, USA), and the ‘Lazcluze + Rybrevant combination 바카라 카지노’ developed by Janssen.
◇OS extended by over 12 months, a "historic milestone"
Reflecting on the announcement of the OS results, Professor Cho, who led the MARIPOSA study for 10 years, remarked, “It felt like giving birth after the pain of labor.” He further shared, “Throughout the global clinical development and the U.S. Food and Drug Administration (FDA) approval process for the Lazcluze + Rybrevant combination 바카라 카지노, I experienced a complex mix of hardship and fulfillment. It was a journey that allowed me to grow professionally and personally.”
“At the beginning of the study, I could not have imagined that overall survival would be extended by more than 12 months,” Cho commented. “The results of the Phase 3 MARIPOSA trial represent a historic milestone, not merely in terms of new drug development, but in their profound impact on significantly prolonging the lives of patients.”
Since receiving U.S. FDA approval in August of last year, the Lazcluze + Rybrevant combination 바카라 카지노 has continued to gain regulatory approval in Europe, the United Kingdom, and Japan, marking its international rollout. Meanwhile, J&J caused a stir in the EGFR-mutated NSCLC ‘first-line treatment’ landscape by unveiling the final OS data from the Phase 3 MAPIROSA trial at the ELCC held in Paris, France, on March 26.
“Until now, aside from Keytruda, there has not been a single drug in the past 100 years that has extended OS by 12 months in the field of lung cancer,” Cho explained. “For patients, an increase in OS by one year is profoundly significant, equivalent to living one additional year out of a typical three-year prognosis, which is comparable in impact of living 20 more years out of an 80-year lifespan,” he added.
◇“Potential for 55-month overall survival… NCCN preferred 바카라 카지노 listing anticipated this year”
In the case of the Lazcluze + Rybrevant combination 바카라 카지노, the mOS has not yet been reached, as more than 50% of the patients in the treatment group remain alive. Cho emphasized the importance of closely observing the ‘OS curve’ for this Lazcluze + Rybrevant combination 바카라 카지노.
“There are indeed patients classified as super responders or hyper responders, whose disease remains stable for several years without progression,” Cho stated. “Taking this into account, the mOS of the combination 바카라 카지노 is expected to reach approximately 55 months.” To further refine this estimate, Cho plans to conduct additional follow-up analyses on the patient cohort to estimate the mOS of the combination 바카라 카지노.
According to Cho, the ‘final piece of the puzzle’ for the Lazcluze + Rybrevant combination 바카라 카지노 is its inclusion as a ‘preferred regimen’ in the U.S. NCCN guidelines. He expressed confidence that, given the current clinical data and momentum, the combination 바카라 카지노 is likely to be designated as a preferred treatment within the year.
At present, the National Comprehensive Cancer Network (NCCN) guidelines recognize both the Lazcluze + Rybrevant combination 바카라 카지노 and Tagrisso + chemo바카라 카지노 as equivalent standard treatment options. Initially, only Tagrisso was designated with the ‘preferred’ status. However, in an update released in December 2024, the NCCN removed this designation.
“Following the publication of the final MARIPOSA study results, the Lazcluze + Rybrevant combination 바카라 카지노 is likely to undergo formal review for inclusion in the NCCN guidelines,” Cho remarked. “There is a strong likelihood that it will be designated as a preferred regimen within the year.” Should this occur, it is expected to be a turning point where the Lazcluze + Rybrevant combination 바카라 카지노 will significantly differentiate itself from Tagrisso and begin widening the therapeutic and clinical adoption gap.
◇Clinical efficacy demonstrated in ‘high-risk subgroups,’ including brain and liver metastasis
The MARIPOSA study revealed that the Lazcluze + Rybrevant combination 바카라 카지노 exhibits enhanced efficacy in patients with a high disease burden. “We were able to clearly differentiate the therapeutic effects of the combination 바카라 카지노 compared to the control group in patients with brain metastasis, liver metastasis, TP53 co-mutations, and ctDNA positivity,” Cho explained. “In these subgroups, the clinical benefit of the combination 바카라 카지노 was particularly pronounced compared to the control group.”
Professor Cho also highlighted the potential for future research expanding upon the Lazcluze + Rybrevant combination 바카라 카지노. “We will soon begin a single-institution prospective study in collaboration with Janssen to investigate the resistance mechanisms of this combination 바카라 카지노,” Cho further noted. “There is also a possibility of conducting studies aimed at applying this combination 바카라 카지노 not only to patients with stage IV lung cancer, but also to ‘high-risk early-stage patients’ following surgical resection for early-stage lung cancer,” he added.

